EXPERIMENTAL
The high-purity closed electrochemical system and experimental conditions were the same as previously reported (1,4) with the exception that the solution was 0.2M NaOH. The working electrodes were a Pt wire (99.99%, 20-miI dla., geometric area = 0.65 cm2 ), an Fe wire (fabricated from three-pass-electron-beam zone-refined iron with less than 4 ppm total metallic impurities, 20-mil dia., geometric area = 0.65 cm2 ), and a similar Fe wire with about 1 cm of 3-miI Pt wire spot-welded to the Fe wire and wound around its tip (Fe » Pt electrode). Each of these electrodes was connected to Pt leads and sealed off in lead-free soft glass tubes. The counter electrode was a large Pt gauze electrode. The reference electrodes were in an arm off the main cell compartment and were a calibrated miniature glass electrode and a Pd wire charged with hydrogen to a potential of about 50 mv positive lo a hydrogen electrode in the same solution. This Pd-H wire electrode was used as the potentiostatic reference.
After the ceil was cleaned with hot concentrated nitric acid it was rinsed for at least 24 hours by continually distilling triple-distilled water into it. About 100 ml of water was then distilled into the cell and cooled. A capsule of sodium metal (99.95% with <55 ppm heavy metals sealed under argon) was broken in two and introduced into the cell under an atmosphere of purified helium. The two halves of the capsule were placed so the open ends were at the bottom of the cell. Taus as sodium metal dissolved, the hydrogen gas generated filled the capsule ends and regulated the introduction of water into the capsule. This allowed a rather slow solution of sodium metal. The resulting sodium hydroxide solution was 0.2 M.
SCHULDINER AND SHEPHERD
Before the Fe electrode was introduced into the cell, a Pt wire cathode was used with the Pt gauze electrode as an anode, and the solution was pre-electrolyzed at about 50 ma for several days under a pure helium atraosphere. Upon completion of the preelectrolysis, the pre-electrolysis cathode was removed, and the helium flow was replaced with hydrogen (purified by passing through heated Pd-Ag tubes). The potentials of the Pt wire, Pt gauze, and Pd wire were then determined against the glass electrode. After the Pt/H2 electrodes reached their equilibrium values and the Pd wire was 50 mv positive to the Pt/H2 potentials, the Fe wire (cleaned in IM HjST^ and "insed with triply distilled water) v;as introduced into the ceil and was made the working electrode. After the open circuit potential was determined, the gauze Pt electrode as the counter electrode and the Pd wire as the reference electrode were connected into the potentiostatlc circuit. The glass reference electrode continuously monitored the potential on the Fe working electrode via a Keithley 610B electrometer. Current flow under potentiostatlc conditions wan determined with a Keithley 601 electrometer and recorded. Potentiostatlc current-voltage curves were then determined after steady-state currents were achieved. Numerous runs under increasing and decreasing potential sequences were determined until reproducible results were obtained.
EXPERIMENTAL RESULTS
The open circuit potential for the Pt, Fe, and Fe » Pt electrodes were each the equilibrium potential of -0.78 v for the H20/H2 reaction in the 13.2-pH, 0.2M NaOH solution. There was no visible sign of corrosion on the pure Fe wire even though the electrode was exposed for several weeks. However, the Fe » Pt electrode did show a very small amount of a yellowish brown corrosion product in the vicinity of the platinum. Tests for iron in solution (thiocyanate) were always negative.
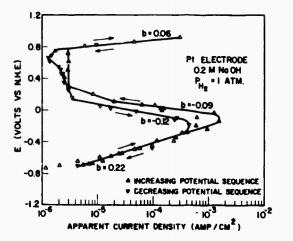
Fig1
The effect of a small area of Pt on the Fe wire was to decrease its activity so that it behaved more like a Pt than an Fe electrode. At low polarizations, however, the Fe » Pt electrode shaved more like the pure iron, eispeclaily in the decreasing potential sequence. The reduction in the limiting current density compared to both the Fe and Pt electrodes is surprising, but probably reflects the small amount of iron corrosion product formed near the Fe/Pt interface. It is evident from these experiments that trace amounts of platinum would have a marked effect on the electrode behavior of pure iron. It i* evident also that, under the experimental conditions using the high-purity system, dissolution of platinum from electrodes during pre-electrolysis or during a run and the subsequent deposition of part of this Pt on the Fe electrode must have been insignificant. There is, in fact, no evidence of platinum impurity affecting the results on the pure iron working electrode. Similarly there is no evidence of traces of iron in solution depositing on the platinum working electrode and affecting its behavior significantly.
上一篇: 晶圆制造工业设施中各种化学物质的定量评估
下一篇: 氮化铝宽带隙半导体及其特性