Experimental
Experiments were performed on two different samples, standard Cu rotating disk electrodes from Pine Instruments ~ 0.95 cm2 ! and blanket Cu/Ta/Si wafer samples ~ 4 cm2 ! obtained from Silicon Quest International. The wafer samples were employed as a comparison to Cu electropolishing on patterned wafer samples that will be reported elsewhere. Electrodeposition from an electrolyte containing CuSO4 , H2SO4 , and benzotriazole was employed to increase the blanket Cu fifilm thickness on the wafer samples to approximately 3-4 m m. The wafer samples were attached to a standard Pine Instruments RDE shaft through a Teflflon mounting sleeve. The Cu/Ta/Si working electrode is illustrated in Fig. 1. The electrical connection is not made through the Si wafer, but around the edges via Cu tape. After the wafer is affifixed, the wafer edges are coated with a thin layer of TorrSeal epoxy.
Results and Discussion
Figure 2 shows typical cyclic voltammograms for a standard Cu rotating disk electrode ~ RDE! and a Cu/Ta/Si wafer sample in an electropolishing electrolyte containing 12 M H3PO4 and 2.7 M C2H5OH at 100 rpm. The difference in the limiting current densities is less than that between subsequent measurements. This demonstrates that despite the nonideal flfluid dynamics and mass transfer near the wafer edges, the Cu/Ta/Si assembly reasonably approximates a standard Cu RDE.15 Active dissolution is seen prior to about 0.75 V vs. SCE, and electropolishing is observed on the limiting current plateau. In some voltammograms, the limiting current plateau is not exactly horizontal due to the sample size, so the inflflection point is taken as the defifinition of the limiting current density.16 This effect is also evident as hysteresis in the voltage at which the limiting current plateau is observed for the Cu/Ta/Si samples.
Cyclic voltammograms were obtained with a variety of different electrolytes of varying H3PO4 and CuSO4 compositions, in addition to some electrolytes to which diluents such as ethanol have been added. In all cases, brightening was visually observed when the limiting current plateau was reached. These diluents are added to obtain a wider range of H2O concentrations for mechanistic studies. In addition, the lower H2O concentrations reduce the limiting current densities. This may be necessary for application of Cu electropolishing to semiconductor processing, which is otherwise diffifi- cult to control, with Cu removal being quite rapid. The electrolytes that were investigated are given in Table I, along with the limiting current density at 240 rpm and the kinematic viscosity.
The effect of the variation of physical properties such as density, viscosity, and the effective diffusion coeffificient within the diffusion layer on a Levich analysis is diffificult to address quantitatively. The most detailed study to date of the effects of nonuniform transport properties was recently published by Barton and West.21 They discuss the effects of the difference between surface and bulk viscosity on the effective diffusion coeffificient obtained from a Levich analysis. Figure 7 show the variation of the viscosity with water concentration for the ethanol-free and ethanol-containing phosphoric acid electrolytes in the present study. In both cases, since the surface water concentration should be close to zero at the limiting current, the surface viscosity is about two to three times greater than the bulk viscosity. In this regime, the effective diffusion coeffificient can be regarded as a good approximation to that at the electrode surface.21
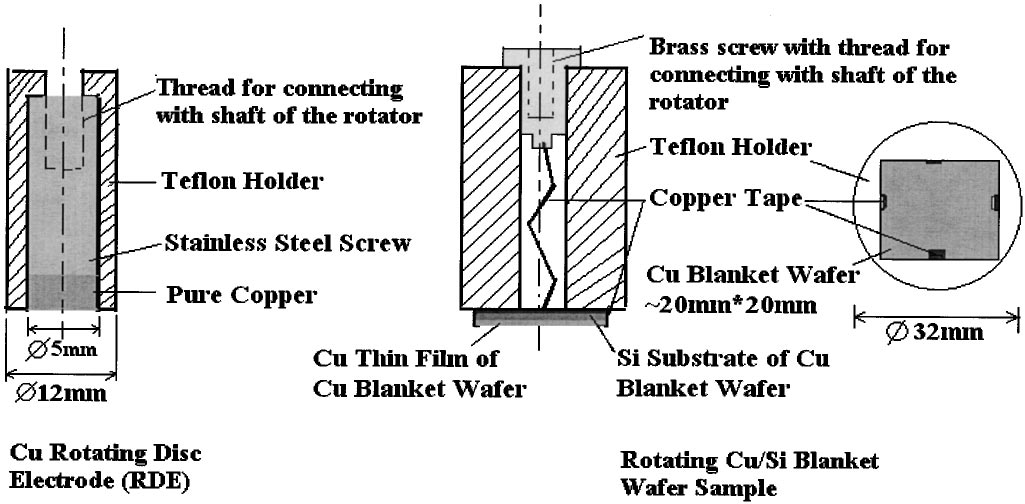
Fig1
Several estimates have been made of the effective diffusion coeffificient of Cu21 in phosphoric acid electrolytes. However, the current study and others demonstrate that water is the acceptor species involved in the rate-determining step. The only available estimate for the water diffusion coeffificient was obtained from the electrohydrodynamic impedance, which does not require knowledge of the species involved, yielding a value of 5 3 102 8 cm2 /s.13 This is in almost exact agreement with the effective diffusion coeffificient determined here when a value of 6 is assumed for sH .
下一篇: 通过旋涂在单晶硅晶片上形成发射极层