1. Introduction
Investigation of the chemical etching of (100) GaAs in a solution consisting of sulphuric acid, hydrogen peroxide and water is of technological and scientific importance [1]. This solution is often used for the preparation of a surface in the sequence of operations in the course of the manufacture of semiconductor devices. For this reason the solution should be optimized; on the other hand, the results obtained constitute a good starting point for the discussion of heterogenous reaction mechanisms.
The sequential chemical reactions taking place on the phase boundary are: the diffusion of reagents from solution to phase boundary; the adsorption of reagents on the solid surface; the oxidation process; transformation of oxidation products to the ionic form; desorption; diffusion of products into the solution.
Reagents in the solutions considered are H202 molecules and H + ions similar to in the H3PO4-H202- H20 system [2]. The flow of reagents to the solid surface depends on their concentration and on solution viscosity, i.e. indirectly on the H2SO 4 concentration. The adsorption generally takes place on the socalled active centres of crystal surface depending on surface orientation. In some cases, etching reveals a crystal structure.
2. Experimental procedure
The range of solution concentrations of the H20- H202-H2SO4 system used in the experiments was limited by the available concentrations of reagents. The composition of the solutions is commonly described by volume percentage on the Gibbs' triangle, its vertices being water, 30 wt % hydrogen peroxide and 96 wt % sulphuric acid. High concentrations of sulphuric acid in the solution result in partial hydrogen peroxide decomposition. To estimate the stability range of the solutions, they were titrated with KMnO4 immediately after the components were mixed, and then after 2 and 24h. If the results of the analyses differed by less than 5% from the calculated compositions and were stable as the same level, the solutions were considered to be stable. The stability line at room temperature corresponds to 50 vol % H2SO 4 without stirring, and a little below this value in stirred solutions, as shown in Figs 1 and 2. All solutions were prepared by mixing proper quantities of H2 SO4 with H20 and cooling to room temperature. H202 was then added slowly to avoid temperature increase. For discussion of the reaction kinetics and mechanism it is convenient to present the solution composition as molar percentage. The range of usable compositions is shown in Figs 3 and 4.
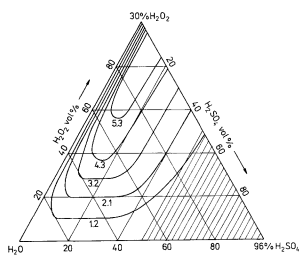
Fig1
3. Results and discussion
The results of etching are presented as lines linking the points of the same etching rates in the field of the Gibbs' triangle and as curves showing the etching rate dependence on one component concentration assuming the concentration of the other components to be constant. The results obtained during etching without stirring are presented in Figs 1.3, 5, 6 and with stirring in Figs 2, 4, 7, 8. Moderately driven stirring enhances the reagent diffusion to/from the phase boundary, thus increasing the dependence on the chemical reaction. Comparison of the results obtained with and without stirring leads to significant conclusions, presented in Figs 9 and 10, in the form of a Gibbs' triangle, in which the fields corresponding to similar surface states and with similar etching mechanisms are indicated.
The shapes of the grooves aligned with [1 T 0], [1 0 0] and [0 1 0] did not depend on solution composition. They were V-shaped with (t 11)A limiting surfaces for the grooves aligned with [1 TO] and U-shaped (with round or flat bottom) for both remaining directions. The change in shape of the [1 1 0] aligned grooves is quite interesting, though difficult to explain. These shapes are seen in Fig. 13. With a fixed concentration of H2SO4 the increase of oxidant concentration is followed by gradual decay of (1 1 1)B walls until the bottom becomes rounded. The increase in H 2 SO 4 concentration causes the (1 1 1)A walls to disappear and the profile also becomes round.
The diversity of groove shapes is accounted for by anisotropy, i.e. the different etching rates of exposed surfaces, in particular (1 00), (1 11)a and (1 1 1)~. It is also connected with the adhesion of masking material to the wafer surface.
Adachi and co-workers [6-10] reported results of wide investigations of the correlation between the shapes of etched grooves and surface orientation, groove direction and type of solution applied. However, he also could find no simple relation between solution composition and groove shape. An analysis of the masking material and the undercutting influence on groove shape was carried in detail by MacFadyene [3].
上一篇: GaN清洗程序分析
下一篇: 光刻胶溶解过程中表面粗糙度的变化