I. INTRODUCTION
The tremendous gain in computational speed and storage capacity afforded by miniaturization of the integrated circuit impels the semiconductor industry forward in its quest for smaller device features. The demands placed on microlithography grow more arduous with each new generation of microprocessors. The minimization of roughness associated with the surface and edges of photoresist images now stands as one of the challenges to continued advances in lithographic technology.
The need for robust lithographic simulators that can model and predict the generation of roughness during photoresist development grows imperative. Most of the earlier simulations of photoresist development do not adequately describe surface roughness because they considered the photoresist a uniform structure. Guerrieri and Neureuther6 have studied the time evolution of the development etch front using a simplifified material crack model in which development proceeds faster along highly exposed fifilaments ~ cracks! than through the background matrix, and they have found that the surface roughness increases with crack length. Trefonas7 alludes to the production of top-surface roughness during his molecular cell-based simulations of percolational development. Scheckler et al.8 have used an even more advanced molecular-scale photoresist development simulation in which a realistic polymer chain length distribution is represented to demonstrate excellent agreement between their model and AFM measurements for the dose dependence of surface roughness. These studies have made signifificant advancements in the simulation of roughness in photoresist development, yet they still rely heavily on empirical data for the dependence of the dissolution rate on such fundamental quantities as molecular weight and degree of deprotection.
II. MODEL DESCRIPTION
A three-dimensional lattice of cubic cells is used to represent the polymer matrix. The number of cells in each orthogonal direction is 73. Each lattice cell, having sides of 0.7 nm in length, corresponds exactly to a single repeating unit of a polymer chain and may have one of the following states: blocked, unblocked, ionized, developed, or void. The initial degree of blocking, specifified by the user, is designated f b0. For a blanket ~ uniform! exposure, the value of f b0 represents the average degree of blocking present in the entire lattice. For a patterned photoresist image, f b0 represents the average degree of blocking prior to exposure; the spatial variation in blocking, f b(x,y,z), prior to dissolution is the product of f b0 and p(x,y,z), a function with values between 0 and 1. The function p(x,y,z) describes the relative amount of protection remaining after exposure and the postexposure bake and is supplied by FINLE Technology’s PROLITH, one of several commercially available lithographic simulators. The initial void fraction, also specifified by the user, is designated f v . The void fraction can represent either the polymer’s inherent free volume or residual casting solvent. A flflowchart describing the steps in the molecular model simulation is shown in Fig. 1.
The average ~ or mean! degree of polymerization is easily obtained from the chain length distribution. In Fig. 4, the average degree of polymerization is plotted versus the maximum degree of polymerization, which is identically equal to the specifified number of steps to be taken in the formation of each chain. The corresponding plot for two dimensions is also shown. The range of chain lengths that is shown here, 10–50 repeat units, is representative of the oligomeric, phenolic polymers used to formulate photoresists. Note that the process produces a linear relationship between the average and maximum degrees of polymerization. The third dimension increases the average degree of polymerization because the extra degree of freedom in the third dimension reduces the average number of redundant steps taken during the random walks and increases the probability for a growing chain to encounter an available cell. The random walk process produces a polymer size distribution that is uniform throughout most of the fifilm, but that has slightly smaller values towards the ends ~ Fig. 5! .
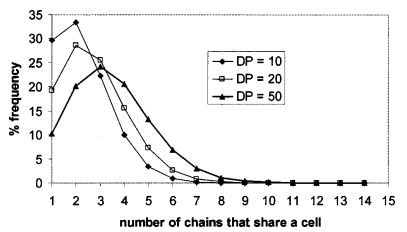
Fig1
Once polymer chains have completely fifilled the grid, the simulator begins the process of ionization. All cells on the top layer of the lattice are always exposed to developer. Ionization is possible for all top-layer cells that are in the unblocked state, and any top-layer cells that are in the void state automatically fifill with developer. Unblocked cells that are adjacent to developed cells may also undergo ionization. Whether ionization actually occurs depends on the probability of ionization (f i), a factor specifified by the user that depends on the concentration of the developer and the pKa of the resin.14,15
The spatial average and the standard deviation of the thickness of remaining photoresist are computed during the simulation. The surface roughness is defifined throughout this article as the standard deviation in the spatial variation of the photoresist thickness. Results for surface roughness, r, and remaining thickness, u , versus time, t, are presented as averages from multiple simulations using different seeds for the random number generator that fifills the lattice. The purpose of running multiple simulations is to sample a larger subspace of the total ensemble of possible spatial confifigurations, and standard deviations of the results are provided to show reproducibility. Dimensionless variables are used wherever possible. Lengths are scaled by the cell height ~ dz! , and time is scaled by the time per ionization/dissolution cycle .
Experimental efforts to determine the effect of polydispersity on the dissolution rate have led to conflflicting results. Tsiartas et al.1 measured the dissolution rates of blends of fractionated novolacs and found that the dissolution rate of the novolac blends decreased with increasing polydispersity. A similar study by Barclay et al.2 concluded that increasing the polydispersity of ~ higher molecular weight! poly~ hydroxystyrene! leads to higher dissolution rates. Figure 10 depicts the predictions from our simulations for the dissolution rate of the chain distributions shown in Fig. 3. According to our model, the lower-polydispersity polymer dissolves faster than the polymer blend, which has a wider molecularweight distribution. In agreement with the conclusions of Tsiartas et al., the simulations suggest that higher molecularweight fractions have a disproportionately large inflfluence on the overall dissolution rate.
上一篇: 硫酸-过氧化氢-水系统中砷化镓的化学蚀刻
下一篇: 改善去除负光刻胶效果的方法