Sensitivity of an orthophosphoric acid, a polyphosphoric acid, -· and an iron (III) chloride etchant to variations in process conditions was examined to determine the potential production - application of the etchant system in the photolithographic fabrication of pure aluminum circuits. The effects of changes in temperature, orthophosphoric acid concentration, polyphosphoric . acid concentration, iron (III) chloride concentration, and dissolved aluminum concentration on etched circuit quality and etch rate were examined. The etchant system allows reasonable variation in preparation and circuit processing without drastically affecting the etched circuit quality. Control of the etchant can be maintained over a broad range of temperatures and compositions.
In the printed circuit industry, chemical etching has long been the accepted method for producing precision patterns in metal films. Etching, in conjunction with present photolithographic technique, affords submicron line resolution.1 Despite the trend to additive "lift-off" techniques for the production of circuit patterns as the pattern dimension order of magnitude approaches the grain size, chemical etching remains an important method of '' pattern production.
For etching of pure aluminum circuits, there are many etchant formulations available. For example, more than 80 etchant formulations are reported in the literature for the etching of pure aluminum roll foil circuits.2 In a· preliminary investigation, 80 such formulations were evaluated on the basis of a reasonable etch rate, uniformly good pattern definition, and positive photoresist compatibility. A later work reports on a spray etchant that gave edge definition variation of +1.27 um.3 This formulation was acceptable but further development was required to find a - formulation that would give more predictable results, reduce dependence on operator skill and experience, and increase the use life of the etchant.
EXPERIMENTAL METHOD
In all cases, 250 mL of freshly prepared mixed acids were placed in a 750 mL beaker. The solution was brought to the desired temperature, then the desired weight of iron (III) chloride was dissolved in the mixed acids. After dissolution of iron (III) chloride, the etch bath temperature was stabilized at the desired temperature, and an aluminum on Kapton (Du Pont) sample mounted -/7 in a specially made polypropylene holder was immersed in the mechanically agitated bath. The sample was positioned with the plane of the sample normal to the etchant flow (Figure 1).
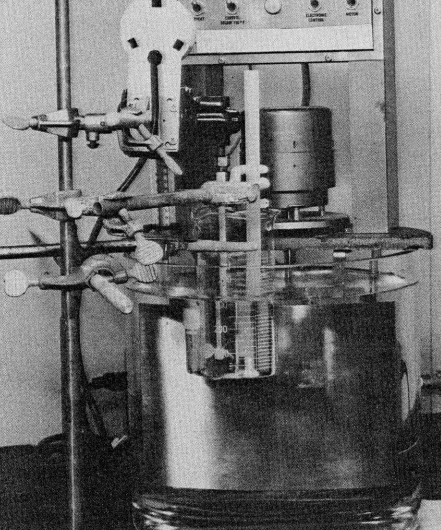
Fig1
RESULTS
An excellent characteristic of the etchant formulation (orthophosphoric acid, polyphosphoric acid, iron (III) chloride) is its stability and the reproducibility of test results. In earlier experiments with standard hydrochloric, nitric and phosphoric - acid etchants, such reproducibility was rare, making analytical characterization difficult.
Effect of Temperature
Although the mechanism of the etching reaction is hitherto unreported, it is desirable to know how the etch rate changes during the etch. In one test, the sample aluminum weight loss was monitored over the duration of the etch period. The rate of etching of aluminum was found to be constant during the etch period, and a linear relationship was exhibited between the sample weight loss and elapsed time (Figure 2).
上一篇: 过氧化氢对铜抛光的影响
下一篇: 微气泡对光刻胶层的影响