ABSTRACT
We review what can be said on wet chemical etching of single crystals from the viewpoint of the science of crystal growth. Starting point is that there are smooth and rough crystal surfaces. The kinetics of smooth faces is controlled by a nucleation barrier that is absent on rough faces. The latter therefore etch faster by orders of magnitude. The analysis of the diamond crystal structure reveals that the { 11 1) face is the only smooth face in this lattice - other faces might be smooth only because of surface reconstruction. In this way we explain the minimum of the etchrate in KOH:H20 in the <001> direction. Two critical predictions concerning the shape of the minimum of the etchrate close to <001> and the transition from isotropic to anisotropic etching in HFHN03 based solutions are tested experimentally. The results are in agreement with the theory.
I. INTRODUCTION
Anisotropic wet chemical etching of single crystalline silicon, gallium arsenide and quartz is one of the key technologies for the fabrication of microsystems. Yet the strong anisotropy of the etchrate in particular etching solutions (e.g. KOH:H20, EDP, TMAH), and the isotropy in others (e.g. HFHN03:HzO) is poorly understood. Mostly the anisotropy of the etchrate is related to chemical reactions on the crystal surface oriented in different crystallographic directions. In this respect maybe the most advanced picture has been proposed by Seidel et al. 111. They assumed that the complex formed by the attachment of an OH-ion to the dangling bond, after the electron has been delivered to the solid state, changes the back-bond energy of the silicon atom with three back-bonds in a different way than iin the situation when one has two OH attached to the silicon atom with two back-bonds. The difficult point however is that the silicon atoms have three backbonds also in the flat (1101 face, not only in the flat { 1111 face; therefore, etchrate and activation energy in these crystallographic directions should be comparable in contrast to experimental evidence.
In this paper we add a number of new experimental results which support the view given here. In particular, we have looked with greater detail at the dependency of the etchrate of silicon etched in KOH on the crystallographic orientation close to the <loo> direction, and we studied the transition to anisotropic silicon etching in HFHN03:CH3COOH. In the next section we briefly review the crystal growth point of view of wet chemical etching. We then describe our experiments and their results. A section of discussion and conclusions follows.
11. THEORY
In kinetics of crystal growth active sites for growth and dissolution play a key role. These active sites are atoms with as many bonds to the crystal as to the liquid (or gaseous) environment. Such a site is called a kink site. An atom in a kink site in a simple cubic lattice is shown in fig. la. The heavy shaded atom has three bonds to the crystal and three bonds to the liquid. In a dissolution situation it is commonly believed that this atom will diffuse over the surface (fig. lb), until it either finds a kink position again or it desorps and diffuses away from the crystal in the liquid phase (indicated in fig. IC). In a growth situation, an atom diffuses from the liquid to the crystal (fig. IC), it diffuses over the crystal surface (fig. 1.b) until it is either desorped or it finds a kink site (fig. la).
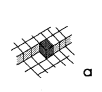
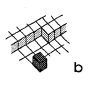
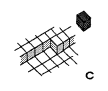
Fig1
This is very much diffeent from the {OOl) silicon face as can be seen in fig. 3. The same operation - creating an adatom-cavity pair - now costs no energy, because one has to break two bonds in order to remove an atom from the (001) face, but one gets them back by placing it back to any position on this face.
It is easily seen that the number of kink sites varies with the crystallographic orientation in a very dramatic way. The perfectly flat { 11 1) face in the diamond lattice has no kink positions (three backbonds, one dangling bond per atom), while on the (001) face of silicon every atom has two back bonds and two dangling bonds - every position is a kink position.
上一篇: 多晶金刚石薄膜技术
下一篇: 金刚石薄膜的等离子体沉积与刻蚀