1. Introduction
Chemical engineering on CuInS2 and Cu(In,Ga)Se2 (CIGSe) absorbers remains a permanent challenge. Thinning of the layers, roughness modifification, surface patterning, elimination of an eventual superfificial layer and adaptation of the surface chemistry for the following processes could be critical for the design of the absorber and then for the ultimate photovoltaic performances [1–6]. Disposing of effificient and reproducible wet etching formulation on I–III–VI must be considered as rather complex and of technological importance as for III–V or II–VI compounds. Beyond the classical empirical approach of etching, the understanding of a wet dissolution process needs specifific study that provides quantitative data on the mechanism and on the related fifinal surface [7,8]. Bromine in aqueous solution is recognized as an effificient oxidizing agent on III–Vs or II–VIs. Previous studies demonstrated that it could be also a suitable etching agent for CIGSe [9,10]. Particularly, it was established that bromine etching permits signifificant decrease of the initial surface roughness providing spectacular flflattening. If the effificiency as etchant, of aqueous bromine formulation is clearly established, few things are available about the knowledge of the process which supports the dissolution. This paper try to progress in this way by considering together kinetic, morphology and chemical parameters. In a previous article, we investigated CIGSe chemical etching with a KBr/Br2/ H2O formulation watching for chemistry and morphology of the resulting surfaces.
2. Experimental details
2.1. Material
The CIGSe layers are prepared by co-evaporation at Wurth Solar [4]. Etchings are performed on 1 × 1 cm2 samples taken from the same layer which present for this work an initial thickness close to 2.7 μm.
2.2. Etching conditions
For this paper the solution HBr: Br2: H2O formulation is 0.25 mol L−1 : 0.02 mol L−1 , ultra-pure deionised water. The dissolution process is performed at 20±0.5 °C using a vertical rotating-disk system at 40 rpm. After etching, samples are rinsed in de-ionized water, dried with nitrogen and maintained under controlled atmosphere when XPS experiments are performed.
2.3. Etching solution analyses During the etching small samples of solution are extracted with calibrated pipet and replaced immediately by the equivalent volume of fresh etching solution. The quantities of the dissolution products at each stage of the dissolution are measured in the set of the extracted samples with a Thermofifisher SOLAAR M6QZ GF-AAS spectrometer. For this work Ga and Cu elements are dosed for each sample [11].
3. Results
The CIGSe absorber is etched from the front side. Firstly the etching kinetic was investigated to determine the dissolution rate. CIGSe samples were etched until the complete dissolution in the aqueous acidic bromine solutions. Solution samples are extracted every 2 min. For each solution sample, titration of Ga and Cu are performed by GF-AAS then accurate evolution of the dissolution process was established which reproducibility for given experimental condition must be pointed out. Starting from the initial rough CIGSe layer, this reproducibility allowed the preparation of estimated thicknesses of flflat CIGSe ranging between 2.4 and 0.5±0.03 μm.
In Fig. 1 we demonstrate that for each element, concentrations of dissolution products linearly increase with immersion time until a steady state value is reached when the entire CIGSe layer is dissolved. The saturation time which is the same for each element depends on sample rotation speed, initial CIGSe thickness, Br2 concentration, bath temperature. For our condition it is typically of 15.7 min. The almost constant slopes of Fig. 1 evidences linear kinetics for the CIGSe dissolution. Slight variations are perceptible always around 7 min, however in the fifirst approximation we can suppose a constant etching rate for the dissolution process. Based on the assumption of a complete dissolution at 15.7 min we can determine a constant rate of 0.17 μm min−1 for the experimental conditions used in this work. So we can affifirm that the etched CIGSe thicknesses are estimated proportional to etch duration.
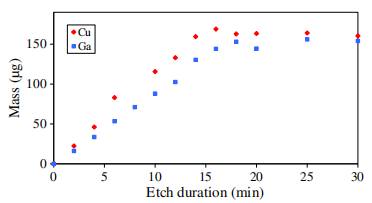
Fig1
4. Discussion
In this paper we propose an attempt to a global understanding of the etching process associated to aqueous bromine solution onto CIGSe. Our multiple experimental characterizations establish that a very stationary etching process is associated with the oxidizing properties of bromine in acidic medium. The quasi linear time dependency of the amount of dissolved products is observed for the fifirst time. It supports the stability and the steady state character of the process which can be considered as constant on the basis of the chemical data provided by our dosage procedure. The linear character of the dissolution induces possible exploitations of very reproducible etching rates as soon as bath engineering is set (Br2 concentration, temperature, hydrodynamic conditions). The global flflattening of the initial surface is the other remarkable trends pointed out in this work. Notably the treatment reduces the peak to valley differences, close to 1 μm in the initial sample (Fig. 3), which might cause problems in the control and homogeneity of the buffer and TCO depositions further down in the processing of the device.
上一篇: 金刚石薄膜的等离子体沉积与刻蚀