I. INTRODUCTIO
Electrostatic discharge causes device destruction through insulation failure and circuit disconnection. This electrostatic discharge occurs when a charged object comes into contact with or into close proximity ta a device surface. In addition, static charge causes the problem of floating particle adhesion [I], [2]. Polymeric materials commonly used to make wafer carriers are natural insulators and easily develop high static charges due to the triboelectric phenomena. This causes floating particles, which are attracted by the static charge of a charged substance, and adhere and accumulate on its surface. In the age of submicron devices,, the particles which need to be controlled will become even smaller. Correspondingly, these finer particles are more sensitive to electrostatic force, and the problem of adhesion becomes more important.
To solve the problem of material charging in air, Nz or Ar inert gas ambience, the use of ionizer and UV (Ultraviolet) light irradiation are effective. In air, ionizers are excellent for the neutralization of static charge. In this method the ions are usually generated by corona discharge, and these ions then neutralize static charge. On the other hand, in inert gas ambience such as N2 or Ar, the static neutralization system employed is UV light which ionizes the N2 and Ar molecules. This method is superior to the static charge neutralization method employing corona discharge for neutralization in inert gas ambience. Additionally this method can be applied to reduced pressure ambience down to - lop3 Torr, demonstrating better neutralization capability than under atmospheric pressure [3], [4].
11. MECHANISM OF STATIC CHARGE REMOVAL FROM THE POLYMER
This experiment was carried out in a Clean Room at a temperature of 20 N 21"C, and a relative humidity of 50 - 60%. Teflon-PFA (4-fluor0 ethylene-perfluoroalkoxy copolymer) and glass were used as charged materials. For the investigation of the static charge removal phenomenon qualitatively, the wafer carrier, container, disk of PFA, and glass plate were rubbed with cotton cloth while wearing insulating gloves (Asahi Chemical Co. Ltd.: Bencot) to charge their surface.
The static charge detector used in this study applied the theory of the Faraday cage. When a charged sample is placed in an isolated conductor, its real static charge appears on the external surface of the conductor. By measuring this static charge, the charge on the sample can be detected indirectly. The schematic diagram and equivalent circuit are described in Fig. 1. The amount of static charge of the sample in the Faraday cage appears across the external capacitor Cm, by then measuring the voltage, V, across the capacitor with an electrometer, the static charge of the sample is obtained using the following expression.
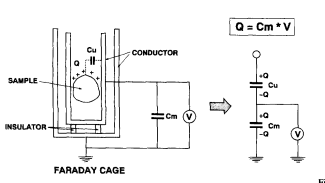
Fig1
Next the amount of triboelectric charge is discussed. Both surfaces of a PFA disk (125 "4, 2 mm thick) were charged up by rubbing with a cotton cloth and the amount of static charge built up was measured using a closed type Faraday cage (see Fig. 2). The number of disks in the Faraday cage was either one or two. In the case of two disks, the amount of initial charge was approximately twice as much as one disk, indicating that the surface area was in proportion to the amount of charge. The amount of charge on the disk reduced gradually, following the attenuation characteristic of potential on the polymer as discussed above.
Charge transfer characteristic for IPA liquid is shown in Fig. IO. The horizontal axis represents applied voltage and the vertical axis represents the amount of charge transfer to IPA liquid. As we found in the N2 gas phase experiment, applied voltage was directly proportional to the amount of charge transfer. However. at the same voltage the amount of charge transfer in IPA liquid was 40 times as large as in a Nz gas ambience. This may be due to the fact that the space between the two electrodes is now filled with IPA liquid which has a high dielectric constant, therefore charge density on the electrode becomes larger.