Wet chemical etching is of enormous relevance to silicon solar cell processing. Conventional acidic etching mixtures consist of hydroflfluoric acid, nitric acid and water. These solutions are widely utilized for saw damage removal, texturing of silicon surfaces for reducing light reflflection and recycling of used solar modules. In addition to these solar material applications, the usage for nanostructured devices has attracted much attention, such as fabrication of silicon nanowires by metal-assisted chemical etching of silicon [1] or tuning the size of silicon nanocrystals with HF-HNO3-H2O etching mixtures [2].
The water concentration is reduced by using fuming nitric acid (100 %) instead of concentrated nitric acid (65 %). High amounts of concentrated sulfuric acid (97 %) additionally bind the water of the concentrated hydroflfluoric acid (40 %) and stabilize nitronium ions in these solutions. NMR and Raman spectroscopic investigations have clearly proven the formation of nitronium ions in etching mixtures with high sulfuric acid concentrations. 14N NMR spectroscopic analysis has shown two discrete signals, which could be assigned to nitric acid at δ = −44.6 ppm and nitronium ions at δ = −132.7 ppm (Fig. 1).
However, Raman spectroscopy allows quantitative NO2 + analysis by evaluation of the intensity of the prominent ν(NO) stretching line of nitronium ions (ν = 1400 cm−1). Fig. 2a shows a typical Raman spectrum of an etching solution containing NO2 + ions. Along with nitronium ions, nitric acid and nitrate ions were identifified as nitrogen containing species. The regression line for quantitative analysis of the nitronium ions was determined by plotting the ν(NO) line intensity vs. the NO2 + concentration of NO2BF4/H2SO4 standard mixtures. Fig. 2b illustrates a calibration plot and the ν(NO) stretching lines of these NO2 + standard mixtures. Due to the temperature dependence of the ν(NO) intensity, all Raman measurements were performed at r. t.
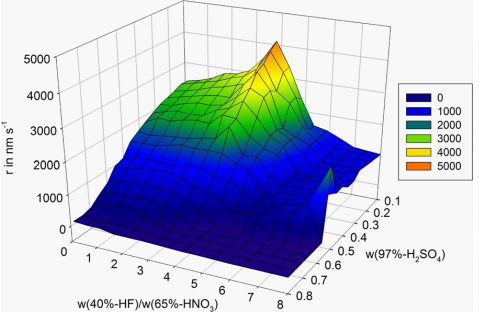
Fig1
For conventional HF-HNO3-H2O mixtures the structures of the etched silicon surface depends on the composition of the mixture. In HF-rich solutions, reaction-controlled processes result in the formation of characteristic oval etching pits. Surface defects, e. g. grain boundaries or dislocation lines, act as starting points for etching reactions. The acceleration of the silicon oxidation by higher amounts of nitric acid leads to smooth surfaces. In these mixtures the diffusion of flfluoride-containing species (F−, HF, HF2 −) to the silicon surface is considered to be the rate-limiting step. Therefore, regions of inhomogeneity are etched preferentially leading to polished surfaces.
下一篇: 湿化学处理后InP表面的研究