All samples were Zn-doped (3 × 1017 cm−3) p-type InP (100) substrates. The samples were ex situ treated in either as prepared 2 M HCl, 1.8 M H2SO4 (50◦C), 15.2 M NH4OH or 2.9 M (NH4)2S solution for 5 min, followed by a 3 min rinse in deionized H2O and blow-dry with N2. The wafers were immediately stored in a N2-purged box and transported to the measurement setup limiting the total air exposure to maximum 5 minutes, including wafer drying.
Surface composition of InP after different wet chemical treatments.— The wettability of InP surfaces after different aqueous treatments, as derived from the contact angle (CA) measurements (Table I), can be used as an indication for the surface termination, since the presence of oxide results in a hydrophilic surface. While for an alkaline NH4OH treatment, a rather hydrophilic surface was obtained with a contact angle below 30◦, both HCl and H2SO4 treatments resulted in a hydrophobic surface (CA ≥ 60◦). The relatively high contact angles suggest that little surface oxide was remaining after immersion into acidic solutions as compared to NH4OH solution.
Due to the extensive amount of oxidized P 2p components determined for an as-received InP wafer, it is clear that this leads to an inadequate starting surface prior to epitaxial growth or dielectric deposition. In order to decrease the amount of elemental (P0) and oxidized phosphorus, different wet chemical treatments were performed. The P 2p and In 3d5/2 spectra of InP surfaces after these treatments are shown in Figure 2, the corresponding spectral weight of the different components are depicted in Figure 3.
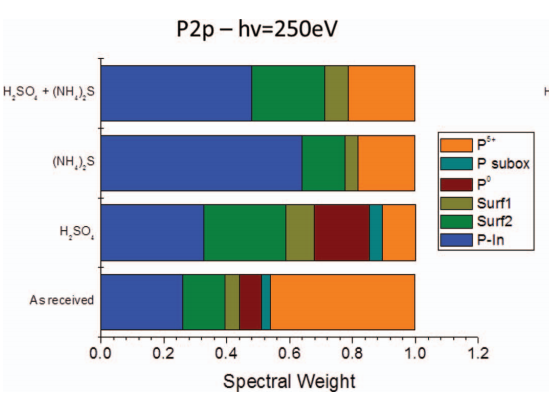
Fig1
Although no roughness (RMS < 2 Å, very similar to as received wafers) is induced during either treatment, a signifificant difference between acidic and alkaline treatment is observed. The morphology after NH4OH immersion is similar to an as-received InP surface, which can be explained by the very low native oxide solubility in this medium. The immersion into acidifified solutions reveals the presence of terraces on top of the surface with lateral dimensions up to 100 nm. For the HCl case, the terraces are more elongated as compared to the more square terraces observed after H2SO4 treatment. This is attributed to a difference in anisotropy in etching.15 The step height between the terraces is 0.29 nm which corresponds to half of the lattice distance. The terraces are therefore either preferentially indium or phosphorous terminated. Due to the much higher solubility of phosphorous oxides in aqueous media,23 it is assumed the surface is indium terminated. This may explain the relatively high indium oxide content observed in the SRPES spectra.
In conclusion, the inflfluence of different wet chemical treatments on the composition of InP surfaces was studied. An as-received InP sample clearly showed the presence of an extensive amount of components. Furthermore, the surface composition and morphology was studied after acidic (HCl/H2SO4) and moderately alkaline (NH4OH) wet chemical treatments. After immersion in a NH4OH solution, native oxides are present which is ascribed to the insolubility of In2O3 at higher pH values. Both HCl and H2SO4 effectively remove the native oxide, although components like P0, In0 and P(2± )+ suboxides remain present. Acidic treatments result in atomically smooth surfaces with the formation of terraces. As alternative surface treatment, the immersion into (NH4)2S was studied. We have shown that (NH4)2S solution results in fewer P 2p components which suggests that a higher quality surface is obtained. Slight etching of the semiconductor in HCl/H2O2 solution followed by native oxide removal, had no signifificant effect on the surface composition.