The reaction stops at the Si surface and leaves the surface hydrogen passivated. This property is of the utmost importance in semiconductor manufacturing and has provided the possibility of removing the native oxide easily and reliably without damaging the underlaying substrate and without leaving a highly reactive and unstable surface.
More recently, Proksche et al. ~ have studied etching in buffered HF solutions as a function of NH~F concentration. They also could discern between two etching mechanisms and fit their data to the model proposed by Judge.
However, the published models agree with the experimental data only in a limited region and only on a logarithmic plot. The proposed models fail to predict the etch rate of dilute HF especially in the region below 2M HE Moreover, the constant introduced in these etch rate equations to improve the fitting has no physical meaning, since according to these models the etch rate is negative for zero HF concentration. Because of this lack of understanding, the engineering of the properties of the HF dissolution of native and thermal oxides has been based up to now on an empirical basis.
For the equilibrium constants/(1 and K2 of, respectively, reaction r~ and r2, the values of 6.85 • 10 4 tool/liter and 3.963 liter/tool are used. ~ These are the equilibrium constants at 25~ and corrected for zero ionic strength. We show that the ionic strength in dilute HF solutions is very low and therefore these values can be used. Moreover, because of the low ionic strength, the calculations can be done with concentrations instead of with activities.
When dimerization is not taken into account, we see from Fig. I that above 0.25M the composition is almost constant and the HF is the most abundant species. The composition is roughly as follows: 90% HF, 4% HF2, and 2% F-.
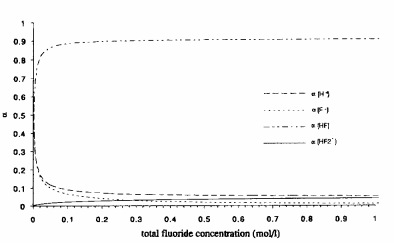
Fig1
From these graphs we see that HF is a very weak acid. Only below 0.05M, HF becomes strongly dissociated, which is according to the Ostwald law, stating that a weak acid becomes strongly dissociated on strong dilution. The dissociation becomes increasingly important below 0.25M HE It has been argued that this weak acidity of HF can be explained by the formation of an ion-pair H30§ -.
In previous calculations, the composition was calculated as a function of the formal HF concentration added to the solution. One can also perform the calculation of a, given a certain fluoride molarity, as a function of the pH. The total fluoride concentration was taken as the fluoride concentration in traditional buffered HF solutions (a 7:1 NH4F/I-IF mixture). The result of these calculations is shown in Fig. 5. From this graph, it is seen that at low pH, the HF is not dissociated and is partially dimerized. At intermediate pH, the HF is mainly transformed into HF2 and at high pH, the mixture contains predominantly the F ion.
上一篇: SiGe的选择性化学湿刻蚀