Recent advances in conductivity control of epitaxial fifilms and availability of high quality bulk ZnO substrates has renewed interest in the ZnO/ZnMgO/ZnCdO heterostructure system for use in UV light-emitters and transparent electronics.1 While the GaN/AlN/InN system is used for these same applications, ZnO has some particular advantages such as relatively low growth temperatures on cheap substrates such as glass and a much larger exciton binding energy 60 meV than GaN 25 meV . The latter is important for achieving robust light emission at high temperatures. The ZnO system also has simpler processing relative to GaN which cannot be wet-etched in conventional acid mixtures at safe temperatures.2-14 There have been a number of recent reports of ZnO-based lightemitting structures involving different approaches, including homojunctions,15-17 hybrid structures involving heterostructures with SiC, GaN/sapphire, AlGaN/sapphire, or Cu2O,18-20 and ZnO p-i-n homojunction diodes grown on oxide substrates.21-23 ZnCdO is an attractive option as the narrow bandgap active region in ZnObased heterojunction light emitting diodes LEDs because of the smaller bandgap of CdO 2.3 eV , providing, of course, that phase separation is avoided during growth of this ternary. Molecular beam epitaxy MBE provides an excellent vehicle for controlling both the purity and phase purity of the ZnCdO.
MBE growth of ZnO and ZnCdO single-layers was performed on c-plane sapphire substrates. Low-temperature Knudsen cells were used to evaporate 6 N purity Zn, Cd, and Mg. Atomic oxygen flfluxes were produced by a radio-frequency rf plasma source. The source was operated at an O2 flflow rate of 2-5 standard cubic centimeters per minute, depending on the Zn flflux, and a forward power of 150 W. In situ reflflection high energy diffraction RHEED was used to monitor MBE growth of the wurtzite phase ZnO and Zn0.95Cd0.05O. After thermal cleaning of the sapphire surface, the substrate temperature was lowered to 400–600°C. The ZnO growth was initiated by simultaneously opening the Zn and the atomic oxygen shutters to ensure Zn-polarity for as-grown fifilms. To reduce the number of variable growth parameters, TS 400°C and Zn/O flflux ratio 1.5:1 were kept constant and only the Cd cell temperature was used to control the composition during growth. After growth, room temperature CL measurements were performed on the samples to defifine the spectral position of the near-bandedge emission peaks for ZnCdO. The Cd composition was verifified by both Rutherford backscattering and the calibrated X-ray photoelectron spectroscopy XPS measurements and was determined to be 4.8 ± 0.3%. The experimental bandgap of our ZnCdO was 2.9 eV from cathodoluminescence CL measurements at 25°C. There was no phase separation in the CdZnO fifilms, as confifirmed by CL emission mapping.
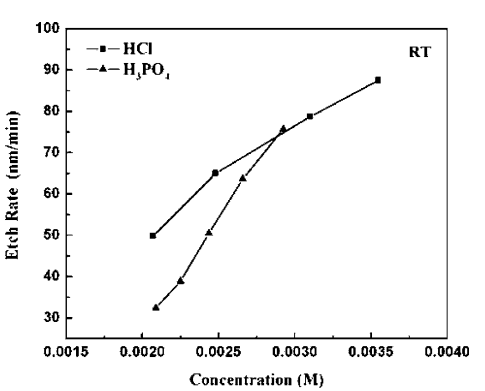
Fig1
Figure 1 shows the etch rates of Zn0.95Cd0.05O as a function of solution concentration for HCl/H2O or H3PO4/H2O at 25°C. Controllable etch rates in the range 100 nm min−1 desirable for mesa formation are obtained over this set of solution concentrations. Note that we used high dilution factors of the acids with water in order to get controllable etch rates. The use of pure HCl or H3PO4 produced very high rates and extensive bubbling in the solutions that led to nonuniform, rough surfaces.
To determine the rate-limiting step in the etching, we measured the etch rate as a function of solution temperature over the range 25–75°C. In dilute mixtures such as used here, it is common that the etch rate is limited by the diffusion of the active etchant species to the ZnCdO surface, or by the diffusion away of the soluble product, i.e., the etching is diffusion-limited. The characteristics include a square root dependence of etch depth on etch time, an activation energy 6 kcal mol−1, and a strong dependence of etch rate on solution agitation. Figure 2 shows an Arrhenius plot of the etch rates of ZnCdO in the two solutions at high dilution factors with water. Under these conditions, the activation energies for etching are in the range 0.4 kcal mol−1, which is consistent with diffusion-limited etching.