As the dimensions of semiconductor devices are downsized, aiming at manufacturing VLSI and ULSI circuits, the cleaning of the silicon surfaces is one of the most critical operations in the fabrication of integrated circuits. The gate oxide electrical characteristics being very sensitive to contaminants, it is essential to study the contamination of Si wafers together with the resulting corrosion process occurring during wet treatment. .
Torcheux et al., and more recently Teerlinck have shown that the rate of elemental copper deposition is approximately proportionnal to the ion-concentration in solution, but decreases slightly with time, the effect being attributed to a diffusion rate determining step. The experiments by Chyan allow the determination of the kinetics of copper deposition from AFM microscopy evaluation. More recently, Parks [14] proposed an electrochemical approach of the process. This idea was elaborated by Chyan [15], who experimentaly demonstrated that electrochemical parameters could be very sensitive to trace amounts of noble metals present in DHF solutions.
In the present work we have undertaken electrochemical and radioactivity measurements to study the mechanism of copper deposition on p and n-type silicon surfaces from DHF solutions containing traces of copper in the range of a few tens of ppb. In order to derive fundamental parameters describing the contamination mechanisms, we have limited the study to systems constituted by electronic grade silicon in contact with high purity deoxygenated DHF solutions, in the dark.
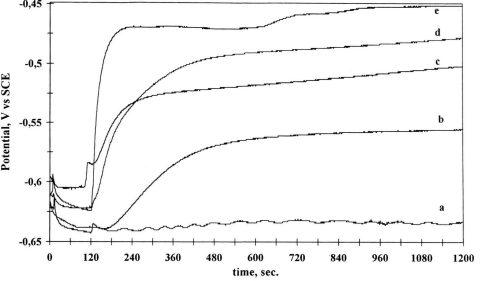
Fig1
T h e addition of trace amounts of copper contaminant was observed to result in a sudden shift of the potential towards positive values, as shown in Figs. 1 (a) and 1(b). Immediately upon copper ion addition the rest potential increased at a rate nearly proportional to the coppe r concentration, and then tended rapidly to a stable limiting value. The reference experimental curve, resulting from the addition of pure deoxygenated DH F under the same conditions, was nearly flat. So, we think that rest potential is characteristic of the silicon surface reactivity modified by the reduction of copper ions, whethe r the silicon substrates were p or n-type doped. According to the usual models for mixed potentials, this variation should be ascribed to a steep increase of the ratio ic / za corresponding to cathodic and anodic site exchange current.
hodic and anodic site exchange current. To explain mor e precisely the shape of these rest potential curves, we have recorded, on each silicon sample, the voltamperometric characteristics, presented in Figure 2. Whe n the current intensity is zero, the curve gives the open-circuit potential which is identical to the final values indicated in Figs. 1(a) and 1(b). This experiment reveals the anodic and cathodic electrode behavior, showing very clearly that the cathodic current is amplified while the anodic current remains nearly unchanged.
下一篇: 半导体湿法化学刻蚀工艺观察