Lithium niobate LiNbO3 is a material widely used in integrated optics because it possesses large nonlinear optical, piezoelectric, and electro-optical coeffificients. For the development of applications such as photonic crystals and Bragg gratings, micropatterning techniques are required which provide high dimensional accuracy, low surface roughness, and a high aspect ratio. One of the most promising techniques is wet chemical etching after ion irradiation IBEE: ion beam enhanced etching . 1-7 During the irradiation, the energy of the impinging ions is transferred to the crystal where it induces the formation of defects. As a consequence, the chemical resistance is reduced and the irradiated regions can be removed by wet chemical etching in HF solutions. Depending on both the irradiation and the etching conditions, the etching rate amounts to 130 nm min−1 for etching amorphized LiNbO3 in 3.7% HF solution at 40°C.6,7 The negligible etching rate of the perfect crystal and the high contrast allow the realization of high aspect ratio microstructures. However, reports on the inflfluence of the etching conditions on the etching behavior and the underlying etching mechanism are scarce.
Commercial congruent x-cut optical grade LiNbO3 crystals were partially masked and irradiated with Ar+-ions at room temperature. To avoid charging and heating of the sample, the current density was kept below 0.3 A cm−2. Multiple irradiation with Ar+-ions of different energies and flfluences were applied as a standard procedure to obtain a uniformly damaged layer from the surface to a depth of 400 nm.6 The computer code SRIM20038 was used to calculate the depth distribution of the number of primary displaced lattice atoms Ndispl z . The ion flfluence NI was converted into a normalized flfluence, the number of displacements per atom dpa ndpa, by ndpa z = NINdispl /N0, N0 denoting the atomic density of LiNbO3 N0 = 9.457 1022 cm−3 . For a normalized flfluence of 1 dpa, energies of 60, 150, and 350 keV each with an ion flfluence of 2.25 1014 cm−2 and 600 keV with an ion flfluence of 1.18 1015 cm−2 were used. Irradiation was performed with normalized flfluences ranging from 0.1 to 0.8 dpa.
Thus, at fifirst a basic understanding of the dissociation of HF in aqueous solutions needs to be achieved in order to describe the relationship between the total HF concentration and the existing composition of the solution. The dissociation behavior of HF in aqueous solutions has been examined by many authors using different methods of measurement.10-15 However, there are substantial disagreements in the interpretation of the results e.g., regarding the existence of the dimer HF 2, the complex formation of H2O with HF to H3O+F−, and the formation of higher flfluoride ions of the type HF nF− with n 1 . Recently, the literature has been surveyed and reanalyzed16 showing that the experimental data concerning the dissociation of HF in aqueous solutions up to 6 mol kg−1 14% at an acid temperature of 25°C can be explained considering only three dissociation equilibria.
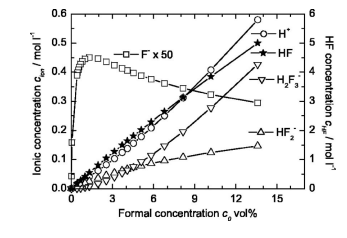
Fig1
The defect concentration nda left scale and the etching rate vetch right scale are depicted in Fig. 2 as a function of the depth z for LiNbO3 irradiated with Ar+ ions at a flfluence of 0.35 dpa and etched in 3.7% HF at 55°C. The defect concentration is constant with nda = 1 from the surface to a depth of 400 nm, which indicates the amorphization of this region. This corresponds to the depth dependence of the calculated normalized flfluence ndpa, which has been included in Fig. 2 for comparison. The etching rate amounts to 170 nm min−1 in the amorphous region and, in accordance with the decreasing defect concentration, decreases to 5 nm min−1 at a depth of 500 nm.
上一篇: HF溶液阳极氧化形成多孔硅层的机理
下一篇: 碳化硅太阳能电池的湿式化学处理