The Enhanced Chemical Cleaning (ECC) process is being developed and investigated bySavannah River Remediation (SRR) to aid in Savannah River Site (SRS) High-LevelWaste (HL W) tank closure. After bulk waste remmoval, the ECC process can be used todissolve and remove much of the remaining sludge heel from Type I and II HLW tanks.The ECC process is similar to the previous chemical cleaning technology (l] in thatoxalic acid is used for both, but the ECC process differs from the previous technology inthe following ways: 1) The sludge heel will be washed with water rather than supernate.2) dilute oxalic acid (.e., or 2 wt) is used in place of concentrated oxalic acid (8wt%); and 3) most of the resultant oxalate is decomposed by an Advanced OxidationProcess (AOP). Reducing the amount of oxalic acid used for dissolution of sludge andthe subsequent oxidative destruction of oxalic acid will lead to a reduction of downstreamimpacts.
Technology gaps to implement the ECC process in the field have been identified. Onesuch gap is the corrosion of the treatment tank (i.e, using an approximately l or 2 wt%oxalic acid solution to maintain a pH of less than 2.0). Corrosion rate data for carbonsteel exposed to the chemical cleaning environment were needed to evaluate the degreeof degradation that could occur in the treatment tank during this process. To date, mostof the corrosion testing has been conducted using 4 wt% and 8 wt% oxalic acid (2]Since the level of corrosion is expected to be a function of the oxalic acid concentration,the treatment tank corrosion rate for l and 2 wt.% oxalic acid need to be determined (3]An upper limit of 2.5 wt.% oxalic acid was selected for the 2 wt.% tests.
The objective of the chemical cleaning process is to dissolve the metal oxides present inthe sludge. while at the same time minimizing the corrosion of the tank wall. Given thatthe iron oxides present initially on the steel surface can mitigate corrosion, these goalswould appear to be contradictory. That is, the oxalic acid could dissolve the protectiveoxide film on the steel surface as well as the iron oxides in the sludge, which would leaveit vulnerable to mmore aggressive attack. Thus a review of the corrosion mechanismm ofcarbon steel in oxalic acid was performed. Similar reviews of the dissolution of metaloxides in oxalic acid(4, 5] have been performed, but are outside the scope of this task.
Corrosion is a process that involves electrochemical as well as chemical reactions. Theelectrochemical reactions differ from the chemical reactions in that for corrosion anexchange of electrons occurs at the interface between the metal and the solution. In orderto maintain a balance of charge, two reactions must occur at the metal surface. The metaldissolution reaction, or anodic reaction, results in the generation of electrons, while thecathodic reaction results in consumption of electrons. An example of these two reactionsare given by equations 1 and 2, where iron is being oxidized to ferrous ions whilehydrogen ions are being reduced such that hydrogen gas is evolved.
The pH and oxidizing power of a given environment determines whether a species isthermodynamically stable in a given environment as shown by Pourbaix diagramms (seeFigures 1 and 2)(6-8]. These potential (E)-pH diagrams present a map of the regions ofstability ofa mmetal and its corrosion products in an aqueous environment. The diagramsalso identify conditions under which l) the metal is stable and will not corrode. 2) solublereaction products are formed and corrosion will occur, and 3) insoluble reaction productsare formed and passivity will occur. The Pourbaix diagram assists in the determinationofregions where the corrosion reaction is possible in a given aqueous environment.
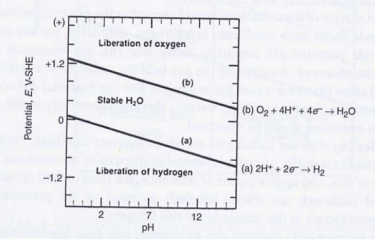
Fig1
One of the weaknesses of the standard Pourbaix diagram is that it does not account forthe presence of other ionic species in solution. Advances and extensions of the diagramshave been made to include other ionic species. The diagram for iron-oxalic acid-water isshown in Figure 3 (9]. Two features have been added to the iron-water diagram shown inFigure 2. The first feature describes the dissociation of the oxalic acid into the hydro-oxalate (HC,04) and oxalate species (see solid red lines on diagram). The distribution ofspecies for oxalic acid is shown in Figure 4 [9]. At a pH < 1.25 oxalic acid is the primaryspecies, while at a pH between 1.25 and 4, the hydro-oxalate species is predominant. ForpH greater than 4, the oxalate species is most stable species. The second feature is theregion of stability for the ferrous oxalate species indicated by the cross-hatched area.This area is defined by the following electrochemical reactions.
Iwo competing processes determine the corrosion rate of steel in oxalic acid: a) the rateof the cathodic reaction, and b) the rate at which ferrous oxalate, the passivating species.forms. The competition between these two processes is illustrated by the followingobservation. Coupon tests were conducted in stagnant, 1 wt.% oxalic acid, at 45 °C and75 °C for two weeks. The corrosion rate for the test at 45 °C was 90 mpy, while for the75 °C test the corrosion rate was approximately 22 mpy. Photographs of the coupons insolution showed a loosely adherent ferrous oxalate film for the 45 C exposure (seeFigure 5). In fact, some of the ferrous oxalate had spalled off of the surface. On theother hand the same figure shows that a tightly adherent ferrous oxalate film in the 75 °Ctest with no evidence of spallation. Initially these results were counter-intuitive giventhat corrosion rates generally increase with temperature.