Conventional manufacturing process of a metal– oxide–semiconductor (MOS) involves various steps such as etching, deposition, CMP etc. During each process, wafer can be easily contaminated by the nano-size particles. These particles on wafer surface might affect the yield reduction. Many literatures reported about wafer cleaning process using physical and chemical method to remove particle effectively. According to the International Technology Roadmap for Semiconductors (ITRS) 2011, critical particle diameter is decreasing to 1x nm with shrinking dynamic random access memory (DRAM) half pitch. Physical cleaning process such as megasonic, jet spray, aerosol, etc., could not prevent pattern damage below 30 nm sized because of its uncontrollable physical force. Therefore, it is essential to clean the surface using wet chemical cleaning process for preventing pattern damage. There are two main factors in wet chemical cleaning process. One is etching of surface and the other is interaction force between particle and substrate. SC1 cleaning mechanism is well known to remove particles on Si substrate representatively. However, particles contaminated on surface not only silicon surface but also many kinds of surface such as poly-silicon, silicon nitride, silicon dioxide. Therefore, it has to be demonstrated particle removal efficiency (PRE) on various surfaces with chemicals based on its etching mechanism and interaction force.
In this study, we investigated critical factor on particle removal using various surface based on silicon substrates with cleaning chemicals which is conventionally used in semiconductor cleaning process. Poly-silicon (poly-Si), thermal silicon-dioxide (Th-oxide) and high temperature silicon-nitride (HT-SiN) were used as substrates for the experiments. Dilute NH4OH (NH4OH:DIW = 1:1000), SC1 (NH4OH:H2O2: DIW = 1:2:50, 60 ºC) and dilute HF (HF:DIW = 1:1000) solutions were used for etching and cleaning test. Etching rate of each substrate was measured using ellipsometer. Zeta-potential was measured to calculate interaction force of each substrate in cleaning solutions. Si3N4 (<1 μm) particles were contaminated on couponed wafer using dipping method for cleaning test. Cleaning process was performed using spin type single tool as following sequence: (1) cleaning 30 sec with cleaning chemicals at 500 rpm (2) rinsing 30 sec with DIW at 500 rpm (3) drying 30 sec with N2 blowing at 1500 rpm.
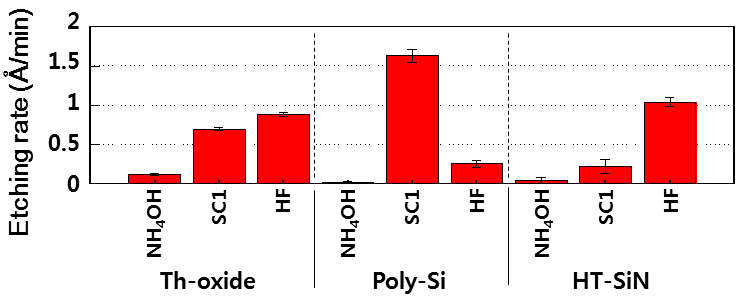
Fig. 1 shows the etching rate of Th-oxide, poly-Si and HT-SiN in cleaning chemicals for 60 min. The etching rate of all the substrates was lowest at dilute NH4OH solution as compared with other solutions. Poly-Si substrate was highly etched by SC1 solution. HF solution has the highest etching rate on HT-SiN substrate. Fig. 2 shows the interaction force between each substrate and Si3N4 particles based on zeta-potential results. pH of NH4OH, SC1 and HF cleaning solutions were 10.8, 10.3 and 2.4 respectively. Repulsive force revealed on Thoxide and poly-Si at pH 10. On the other hand, HT-SiN appeared attraction force at all the conditions. Based on results of etching rate and interaction force, PRE was evaluated as shown in Fig. 3. PRE of Th-oxide was higher at dilute NH4OH solution and SC1 solution. PRE was affected by etching of Th-oxide surface and strong repulsive force between Th-oxide surface and particles in alkaline solution. In spite of high etching rate in HF solution, it could not remove particles effectively due to particle recontamination by attraction force in acidic condition. PRE of Poly-si was similar with Th-oxide in alkaline solution. This means that Th-oxide was affected by interaction force as critical factor for wet chemical cleaning. PRE of HT-SiN surface increased with increasing etching rate. Therefore, etching rate is the most critical factor than interaction force on HT-SiN surface.
Thus, each substrate has dominant factors in wet chemical cleaning. It may be etching rate or interaction force or both of them. If these factors are considered when conducted cleaning of various substrates, the substrates will be cleaned effectively.
上一篇: 高效光伏硅片制造中的水基超声化学清洗
下一篇: 湿化学蚀刻分离硅衬底的镓极性氮化镓层