Hydrogen peroxide (H2O2 ) is a clear, colorless liquid that is completely miscible with water produced on a massive scale through the anthraquinone auto-oxidation (AO) process developed by Riedl and Pfleiderer in 1939 at I. G. Farbenindustrie.[1] Despite being known since the first synthesis of eau oxygen8 e in 1818 by Th8 nard through the acidification of barium peroxide (BaO2 ) with nitric acid,[2] its numerous beneficial properties are still being discovered today. For example, unlike many other types of anti-infective drugs or biocides, the general mechanisms of action of H2O2 significantly reduce any risk of the development of resistance to the biocide over time,[3] which opens up the possibility of its widespread utilization as an antimicrobial chemical in both developed and developing countries.
Serious environmental concerns (including the formation of dioxins and other deleterious chlorinated products) led, over the course of the second half of the 1990s, to the replacement of chlorine with H2O2 as a bleaching agent in paper production.Since 2008, in addition, H2O2 started to be extensively used as a terminal oxidant in the newly industrialized synthesis of propene epoxide (PO). In the mid-1990s, the world’s global capacity (based on 100% H2O2 ) was around 1.5 million tonnes per annum and a world-scale plant had a capacity of around (20 000–40 000) . As of early 2015, the global capacity had reached 5.5 million t , with 300 000 plants built to support PO synthesis. By doing so, H2O2 turned from being an expensive specialty chemical into a large-scale commodity, plentiful and affordable, opening up the route to several new uses of this valued substance, which are highly beneficial to the environment. Perhaps unsurprisingly, research into H2O2 was reinvigorated, leading to the introduction of interesting new materials and innovative processes based on this valued molecule.
Herein, we review the production and use of H2O2 as a green industrial oxidant to provide an overview of the explosive growth of the industrial use of H2O2 over the last three decades and of the state of the art in its industrial manufacture, with important details of what determines the viability of direct production from O2 and H2 compared with the traditional AO process.
According to a leading practitioner of the industry, in 2009 the variable cost of production (raw materials, electricity, and steam consumption) in modern plants was typically $300 H2O2 (on 100 wt% product basis), the fixed costs of production around $100 for maintenance and operation, and the capital cost around $300 , with sales price varying from $700 to 1200 , depending on the amount sold.
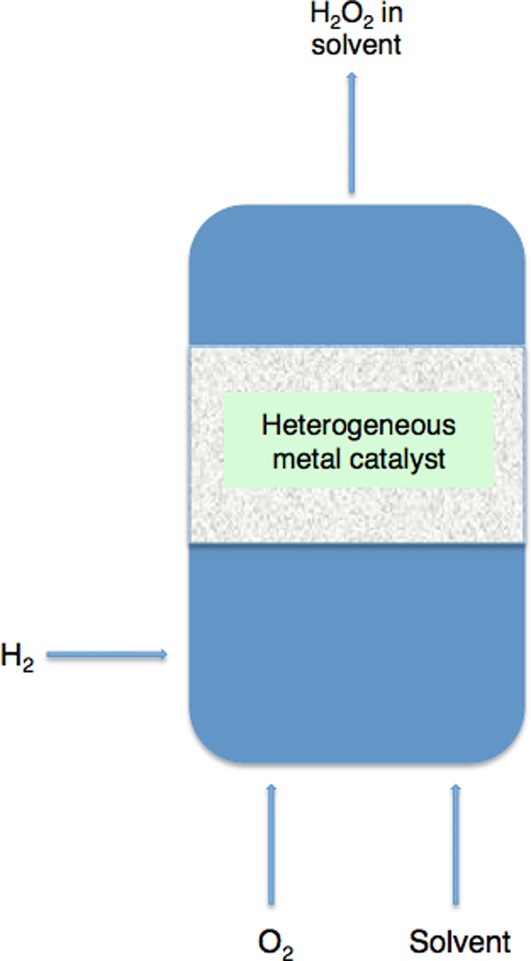
Fig1
In 2007, the company that developed the PdPt/C-based catalytic process claimed that the manufacturing process based on direct synthesis would involve up to 50% lower capital cost and 20% lower H2O2 cost.[ However, such figures were questioned in a subsequent comparative study published in 2013 by the same market research analysis company, which evaluated the Qu8 bec direct synthesis mentioned above. According to the latter research, the OPEX and CAPEX for a standalone 98 kt direct synthesis plant based on such a catalyst were estimated to be about 25% higher and 33% lower, respectively, compared with a typical anthraquinone-based plan.
下一篇: 二氧化硅薄膜的化学蚀刻