The bonding energy plays a significant role in wafer bonding. The temperature dependence of the bonding energy has been studied to understand the physical/chemical interaction at the bonded Si/Glass interface for a variety of glasses. Several primary glass attributes, such as surface roughness, Young’s modulus, strain point, CTE, and glass constituents, have been evaluated to correlate to the bonding energy. While the other attributes have been proven insignificant to the bonding energy, notable correlations with the glass surface roughness, the strain point of the glass, and the alkaline content in the glass have been discovered.
In hydrophilic silicon-to-silicon wafer bonding, it is known that the bond can be significantly strengthened at a temperature above 200 ºC, and the bond is completed at approximately 1100 ºC by forming covalent siloxane bonds 5 . According to the experiments, in contrast, Si/Glass bonded pairs complete the bonds at lower temperatures – 150 ºC to 350 ºC, depending upon the type of glass. The bond energies of the silicon wafers bonded to a variety of glasses, such as Soda Lime, Corning® 1737, Corning EAGLE XG® glass, Corning JadeTM glass, SCHOTT Borofloat® , Corning Pyrex® , and Quartz, have been characterized as a function of annealing temperature. The primary glass attributes, such as Young’s modulus, surface roughness, CTE, strain point, and glass constituents, have also been evaluated to clarify the correlation with the bonding energy.
6-inch silicon and glass wafers were used to make bonded pairs for the measurements. The silicon wafers were all prime (100) p-type and single-side polished. Some of the glass wafers were polished, while Corning 1737 glass, EAGLE XG glass, and Jade glass were non-polished due to the fact that they were originally formed in sheets by Corning’s proprietary Fusion-Draw Process and cored into wafers afterwards. The surface roughness of the glass was characterized before and after the wafer clean process. Both silicon and glass wafers were cleaned respectively right before bonding with each other. The bonding energy was then characterized at room temperature. Afterwards, the bonded pair was annealed at elevated temperatures incrementally. In between each two annealing process, the bonding energy was re-measured until the razor blade broke the glass during the measurement.
After the silicon was bonded to the glass, the bonded pair was loaded on a customized atmosphere anodic bonder instead of a hot plate. The bonder consisted of top and bottom heating plates. During the annealing process, both plates were touching the bonded pair with moderate force which is just enough to hold the pair. Therefore, the temperature distribution across the bonded pair was very uniform, and the temperature ramps were very steady and controllable. The temperature over-shoot was always less than 3% of the target temperature. There was no voltage applied to the annealing process.
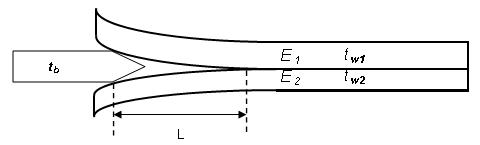
Fig1
After both the silicon and the glass wafers were cleaned, they were bonded at room temperature. The bonding energy was then measured for each bonded pair at room temperature, after annealing at 100 ºC, 150 ºC, 200 ºC, 250 ºC, and 300 ºC. For most of the bonded pairs, the bonding energy has exceeded the glass elastic energy below 300 ºC. As a result of that, the glass substrate broke as the razor blade was attempted to crackopen two substrates. In that case, the bond was considered “completed” with that certain glass thickness. The glass thickness was either 0.5 mm or 0.63 mm, and the bonding energy was calculated accordingly. The last data point before the glass broke was the highest measurable bonding energy unless a thicker glass substrate was used.
上一篇: 选择性湿蚀刻和腐蚀工艺的基础研究
下一篇: 无掩膜紫外光刻技术