Mrofluorocarbons (CFCs) and other halogenated solvents for precision cleaning of metals, plastics, composites, ceramics, electronic components, and other materials. In 1991, for example, the Environmental Protection Agency estimated that approximately 170 million pounds of CFC-113 and methyl chloroform were used by US. industries for vapor degreasing operations.' The use of these solvents is being phased out because of their role in stratospheric ozone depletion, and a number of alternative cleaning technologies and solvents are in various stages of development and use.
Many of these alternative technologies are limited in their application and present new problems, including high solvent and operating costs (e&, estimates for perfluorocarbon solvents are as high as $15/lb); toxicity (e& VOCs and HCFCs); flammability (e& hydrocarbons, alcohols, and ketones); and the generation of wastewater streams (aqueous and semiaqueous systems). No single solvent alternative or cleaning technology developed to date has been found to have the near-universal application of CFCs for precision cleaning applications.
Many of these alternative technologies are limited in their application and present new problems, including high solvent and operating costs (e&, estimates for perfluorocarbon solvents are as high as $15/lb); toxicity (e& VOCs and HCFCs); flammability (e& hydrocarbons, alcohols, and ketones); and the generation of wastewater streams (aqueous and semiaqueous systems). No single solvent alternative or cleaning technology developed to date has been found to have the near-universal application of CFCs for precision cleaning applications.
Supercritical carbon dioxide (CO,) has been identified as a promising solvent alternative to CFCs for a variety of precision cleaning applications, primarily because of its low cost, low toxicity, nonflammability, and environmental acceptability. This article presents recent experience working with CO, as a cleaning solvent to reduce the technology to commercial practice.
The use of supercritical fluids (SCFs) in precision cleaning grew out of previous developments in the technology over the last decade. The novel solvent properties of SCFs, in particular CO,, have been exploited commercially in the food, pharmaceutical, and petroleum industries. More recently, SCF technology has been commercially applied to the extraction of hazardous organic chemicals from industrial wastewater. At present, CO, is being investigated for a number of precision cleaning applications, and the first commercial systems are in operation.
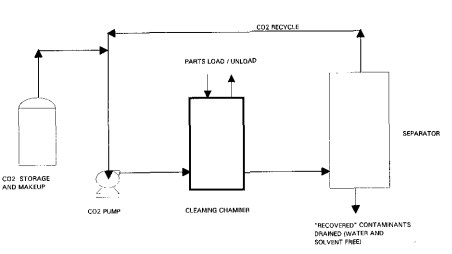
Fig1
Spica1 operating temperatures and pressures range from ambient to 60°C and 750--3,000 psi. Temperature- and water-sensitive surfaces or substrates are often good candidates for cleaning with CO,, whereas pressure-sensitive components may be damaged. Pressure sensitivity can be controlled in some applications by changing the rate of pressurization and/or depressurization.
The process schematic in Figure 1 illustrates the hasic batch cleaning operation. Materials to be cleaned are loaded into the cleaning chamber using an appropriate basket, container, or fixturing device. The chamber is then sealed using a quick-opening closure mechanism designed for the operators and working environment.
上一篇: 高效太阳能电池的晶圆清洗程序比较