This is a preprint of a paper intended for publication in a journal or proceedings. Since changes may be made before publication, this preprint should not be cited or reproduced without permission of the author. This document was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, or any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for any third party’s use, or the results of such use, of any information, apparatus, product or process disclosed in this report, or represents that its use by such third party would not infringe privately owned rights. The views expressed in this paper are not necessarily those of the United States Government or the sponsoring agency.
Small nanoparticles have a large proportion of their atoms either at or near the surface, and those in clusters are essentially all on the surface. As a consequence, the details of the surface structure are of paramount importance in governing the overall stability of the particle. Just as with bulk materials, factors that determine this stability include “bulk” structure, surface reconstruction, charge balance and hybridization, ionicity, strain, stoichiometry, and the presence of adsorbates. Needless to say, many of these factors, such as charge balance, hybridization and strain, are interdependent. These factors all contribute to the overall binding energy of clusters and small nanoparticles and play a role in determining the deviations from an inverse size dependence that we have previously reported for compound semiconductor materials. Using first-principles density functional theory calculations, we have explored how these factors influence particle stability under a variety of conditions.
The binding or cohesive energy of nanoparticles is of importance because it determines a wide range of physical properties including melting point, congruent vapor pressure, sintering rate and solubility. This is especially true in the case of smaller particles where these properties are often difficult to determine experimentally. Fortunately, first principles theoretical calculations using, for example, density-functional theory can be used to obtain nanoparticle energies with a reasonable degree of accuracy. From these theoretically obtained energies, many relevant physical properties can be estimated to supplement the experimental situation. Furthermore, such calculations can now be performed relatively easily, particularly in the small size range where experimental determinations are most difficult.
Previously, we have shown that, unlike most metals, the per-atom-pair binding energy of compound semiconductor clusters and small nanoparticles is not a linear function of the inverse of the particle diameter or radius . It is, rather, closer to a quadratic dependence, and the per atom-pair binding energy approaches that of the bulk material more rapidly with increasing size than for most metals. This behavior is similar to that of the elemental semiconductors, C, Si and Ge, and of the Group IV metals, Sn and Pb. Interestingly enough, it also resembles the non-linear inverse size dependence seen for metal oxides.
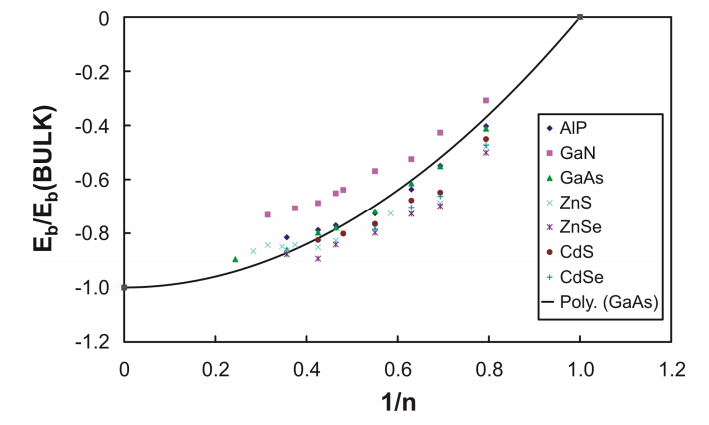
Fig1
To summarize our earlier results, in Figure 1 are shown the normalized, per atom-pair (M-X) binding energies as a function of 1/n for AlP, CdS, CdSe, GaN, GaAs, ZaS, and ZnSe. In order to show all of the data on the same graph, they are plotted as a function of both reduced energy and reduced reciprocal radius. Normalization of the energy along the y-axis was achieved by dividing the particle per atom-pair binding energies by that for the bulk, and referencing that to the energy for a single pair of M-X atoms. In addition, we have, in essence, normalized the reciprocal size along the x-axis as well by using 1/n, where n is the cube root of the number of atom-pairs in the cluster or nanoparticle, as our variable. Here, n = r/r0 where r is the effective radius of the particle and r0 is the average radius of a single pair of M-X atoms. Noting that, at least for low aspect-ratio particles, n scales with r, the radius of the particle, this graphic representation is equivalent to plotting the per-atom binding energies as a function of the reduced inverse size of the particles. In Figure 1, the point on the far right of the diagram refers to a single atom-pair (n = 1) while the point on the far left refers to the bulk material (n = ∞ ).
上一篇: 化合物半导体射频器件的数值分析
下一篇: 化合物半导体纳米线光电探测器