Multilayer dielectric (MLD) diffraction gratings are essential components for the OMEGA EP short-pulse, high-energy laser system, so they must have both high optical-diffraction efficiency and high laser-damage threshold. The cleanliness of optical surfaces intended to be deployed in high-peak-power laser systems is of paramount importance, and the fabrication of these MLD gratings involves processes that utilize a wide variety of both organic materials (photoresists, photoresist solvents, and photoresist developers) and inorganic materials (metals and oxides of various cationic elements) that may remain behind either on the surfaces or in the grooves of the MLD structure after processing. Because a substantial number of these materials can have significant optical absorbance, the incomplete removal of these residues puts the MLD gratings at an increased risk of experiencing catastrophic laser-induced damage. Although there exists a certain amount of anecdotal and empirical evidence as to the effectiveness of certain wet chemical cleaning processes, which appear to be effective in removing trace residues from grating manufacturing, there does not exist to date a truly systematic study that strives to relate the chemical composition of contaminants introduced during the fabrication process of “structured” optical components (such as MLD gratings) with laser-induced damage. To this end, we have investigated the effectiveness of a number of wet-chemical cleaning processes currently used by the semiconductor industry for cleaning LLE-fabricated MLD gratings. The goal of this investigation was to identify a process or processes that were sufficiently aggressive in the removal of residual processing contaminants but not so aggressive as to produce physical/chemical damage to the MLD grating structure that would reduce its high diffraction efficiency.
Hydrozone Process: Hydrozone+,4 developed as a replacement for Piranha clean, uses ozone gas dissolved in DI water. An aqueous solution at elevated temperatures is sprayed across a surface while dry ozone gas is admitted into the cleaning chamber. The ozone diffuses through the thin boundary layer of water, in which the water hydrolyzes the organic bonds, making them susceptible to attack by O3. The elevated water temperature maximizes the reaction rate. The reaction by-products (CO2 and H2O) and resist fragments are carried away in the boundary layer of water.
The Nanostrip process was evaluated at different immersion times to see how this affected the laser-damage threshold. The laser-damage threshold was found to decrease with increased immersion time. This decrease in damage threshold could be due to re-deposition of organics on the surface since this work was performed in a static (un-agitated) lab-scale bath. Similarly.
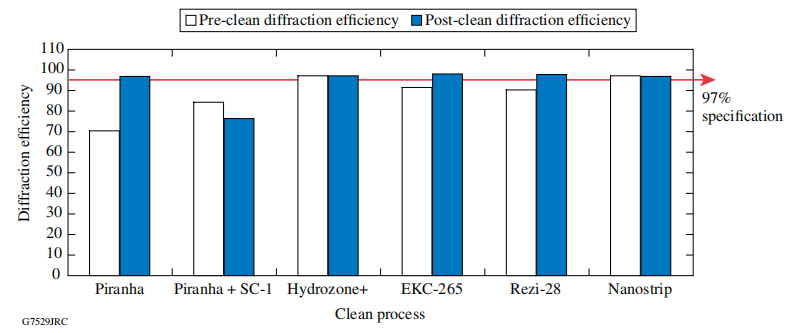
Fig. 108.42
The species predominantly associated with the SiO2 grating are indicated in Fig. 108.42. Samples that have high Si-related ion peaks indicate that the surface is relatively clean since the top grating surface is SiO2. The lack of Si-related ion peaks indicates that there were other contaminants on the surface. As shown, the Piranha and Hydrozone+ clean samples had a high signal for Si and SixOy species, indicating that there is less contamination. The MLD O2 ion-etch clean sample does not show any signal for Si or SixOy species. This indicates that there was a layer of other contamination on the SiO2 surface. The species associated with the MLD O2 ion-etch clean sample are mainly metals, which could be originating from contamination within the etch and ash chambers (shown in Fig. 108.43). Most of these metals, however, were removed during the cleaning process.
Using 100-mm-diam MLD gratings fabricated at LLE, we evaluated different cleaning methods designed to optimize both optical diffraction efficiency and laser-damage threshold of these gratings for the OMEGA EP Laser System. Pre- and post-clean diffraction efficiency and laser-damage threshold were measured for each of the samples. Scanning electron microscopy (SEM) images were collected and analyzed to understand if any visual surface contamination existed after cleaning. Additionally, a baseline time-of-flight secondary ion-mass spectrometry (ToF-SIMS) and shallow-depth profile analysis was performed to understand the type of contamination remaining after the different process steps.